Corrosion of Embedded Metals in Concrete
Corrosion of reinforcing steel and other embedded metals is the leading cause of deterioration in concrete. When steel corrodes, the resulting rust occupies a greater volume than the steel. This expansion creates tensile stresses in the concrete, which can eventually cause cracking, delamination, and spalling (Figs. 1 and 2).

Steel corrodes because it is not a naturally occurring material. Rather, iron ore is smelted and refined to produce steel. The production steps that transform iron ore into steel add energy to the metal.
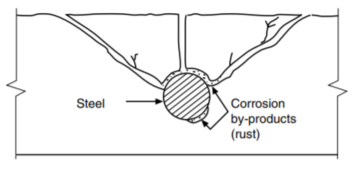
creates tensile stresses in the concrete and cracking
Steel, like most metals except gold and platinum, is thermodynamically unstable under normal atmospheric conditions and will release energy and revert back to its natural state—iron oxide, or rust. This process is called corrosion.
For corrosion to occur, four elements must be present: There must be at least two metals (or two locations on a single metal) at different energy levels, an electrolyte, and a metallic connection. In reinforced concrete, the rebar may have many separate areas at different energy levels. Concrete acts as the electrolyte, and the metallic connection is provided by wire ties, chair supports, or the rebar itself.
Corrosion is an electrochemical process involving the flow of charges (electrons and ions). At active sites on the bar, called anodes, iron atoms lose electrons and move into the surrounding concrete as ferrous ions. This process is called a half-cell oxidation reaction, or the anodic reaction, and is represented as:
The electrons remain in the bar and flow to sites called cathodes, where they combine with water and oxygen in the concrete. The reaction at the cathode is called a reduction reaction. A common reduction reaction is:
To maintain electrical neutrality, the ferrous ions migrate through the concrete pore water to these cathodic sites where they combine to form iron hydroxides, or rust:
This initial precipitated hydroxide tends to react further with oxygen to form higher oxides. The increases in volume as the reaction products react further with dissolved oxygen leads to internal stress within the concrete that may be sufficient to cause cracking and spalling of the concrete cover. Corrosion of embedded metals in concrete can be greatly reduced by placing crack-free concrete with low permeability and sufficient concrete cover.
Concrete and the Passivating Layer
Although steel’s natural tendency is to undergo corrosion reactions, the alkaline environment of concrete (pH of 12 to 13) provides steel with corrosion protection. At the high pH, a thin oxide layer forms on the steel and prevents metal atoms from dissolving. This passive film does not actually stop corrosion; it reduces the corrosion rate to an insignificant level. For steel in concrete, the passive corrosion rate is typically 0.1 µm per year. Without the passive film, the steel would corrode at rates at least 1,000 times higher (ACI 222 2001).
Because of concrete’s inherent protection, reinforcing steel does not corrode in the majority of concrete elements and structures. However, corrosion can occur when the passivating layer is destroyed. The destruction of the passivating layer occurs when the alkalinity of the concrete is reduced or when the chloride concentration in concrete is increased to a certain level.
Read Also
