Carbonation is the term used to describe the effect of carbon dioxide on a material. The phenomenon rarely leads to structural problems but carbonation-induced corrosion can lead to unsightly spalling on structures.
The repair costs, for example to multi-storey office development façades, can be considerable. Carbonation of cementitious materials results in a lowering of the pH – making the material less alkaline – and hence the term ‘neutralisation’ is also sometimes used.
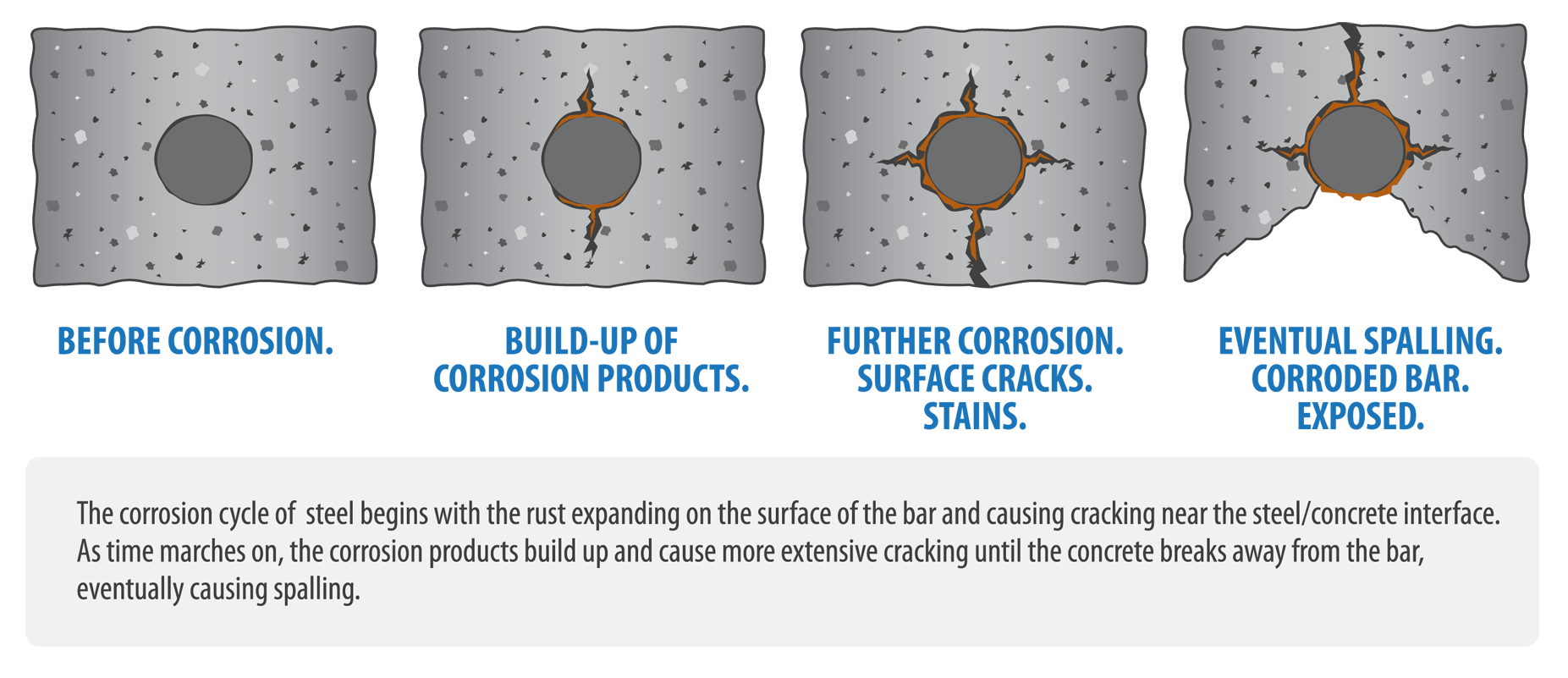
Reinforcement in concrete is embedded in an oxygenated alkaline solution. The reinforcement will not corrode if the protection afforded by the passive film – a thin layer of oxide deposited on the steel – remains substantially intact. This insoluble oxide film prevents oxygen reaching the steel and inhibits corrosion. The reinforcement is said to be ‘passive’ when it is in this state. Corrosion of reinforcement can commence however if the passive oxide film protecting the reinforcement is destroyed, the cover concrete is sufficiently permeable to oxygen and moisture, and the concrete is moist enough to serve as an electrolyte. The lowered pH in zones of carbonated concrete may threaten the continuity of the passive film. It is important therefore to specify cover concrete that is capable of resisting the penetration of the carbonation front as far as the reinforcement during the service life of the structure.
The pH of the pore solution of fresh concrete is approximately 12.6. The alkaline nature of concrete is principally due to the presence of calcium hydroxide, Ca(OH)2, formed during the hydration of cement. Dissolution of the calcium hydroxide leads to the presence of hydroxyl ions in the pore water and this gives concrete its high pH. The calcium hydroxide is susceptible to reaction with carbon dioxide from the atmosphere. The reaction proceeds in the presence of moisture as water provides a medium for the reaction.
Concretes in service will almost always contain sufficient moisture for the reaction to proceed. The reaction involves the production of calcium carbonate (CaCO3). Conversion to calcium carbonate influences the surrounding pore fluid pH, which falls to about 8.3. This fall in pH results in carbonation front which divides carbonated and non-carbonated zones. While carbonation does not adversely affect the concrete itself the durability of reinforcement may be compromised. This is because the passive ferrous oxide layer on the reinforcement breaks down when the surrounding pH falls below 9.